Who Else Wants Tips About How To Tell If A Solution Is Unsaturated
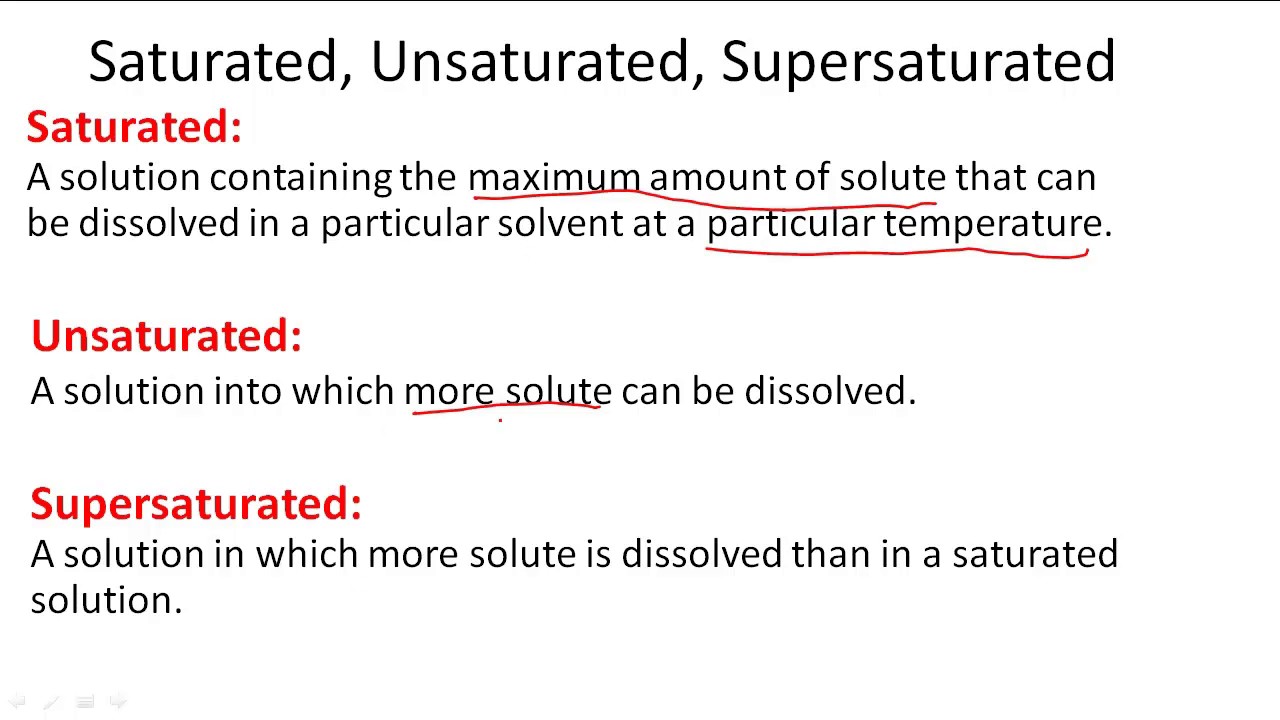
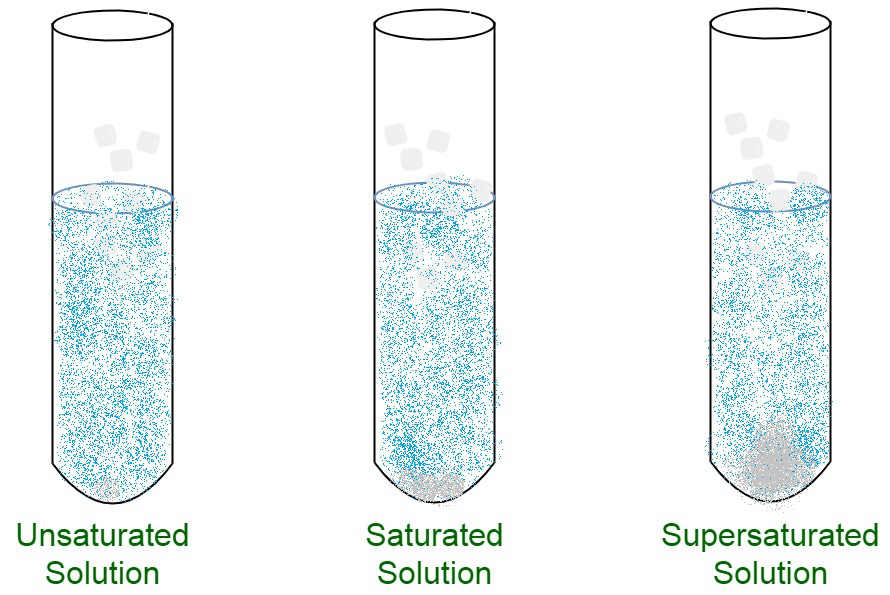
An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved.
How to tell if a solution is unsaturated. Alkaline potassium permanganate test (baeyer’s test) bromine test. If more solute is added and it does not dissolve, then the original solution was saturated. There are two methods for detecting the unsaturation in an organic compound.
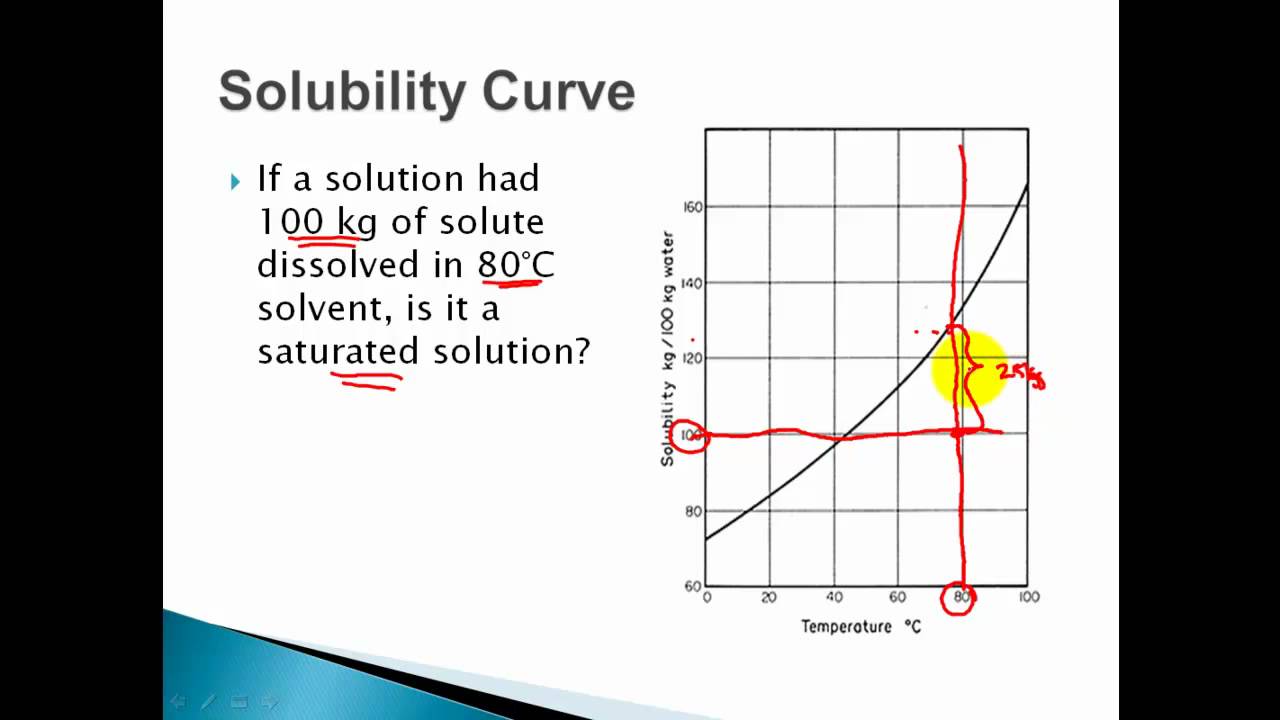
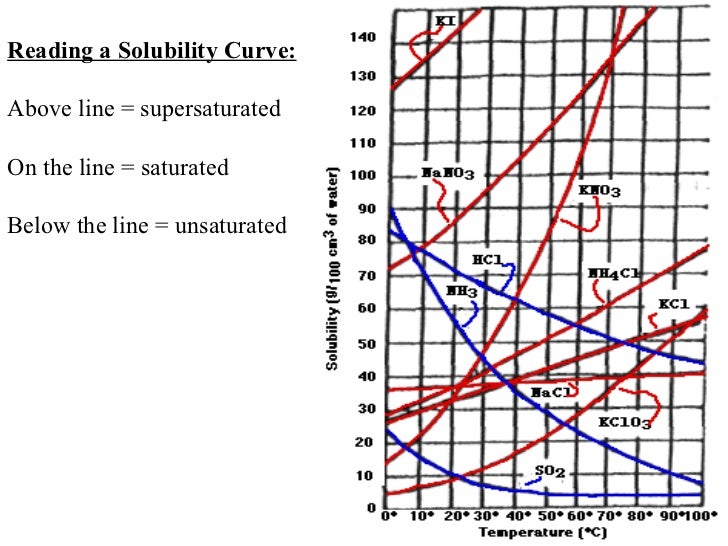
Learn if a solution is saturated or unsaturated by reading a solubility curve.example questionswhat mass of solute will dissolve in 100g of water at the foll. A solution of this composition is also described as a saturated solution since it can accommodate no more kcl. 317k views 11 years ago.
If a solution contains less than the maximum amount of solute, it is unsaturated. The molecular formula of a. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature.
At 20°c, the maximum amount of nacl that will. How can you tell if a solution is saturated or unsaturated? Carbon and its compounds > saturated and unsaturated carbon compounds.
How can you tell if a solution is saturated or unsaturated? A solution with the maximum possible amount of solute is saturated. We talk about saturated and unsaturated solutions (and what they are!) we also figure out how to calculate whether a solution is saturated or unsaturated.thi.
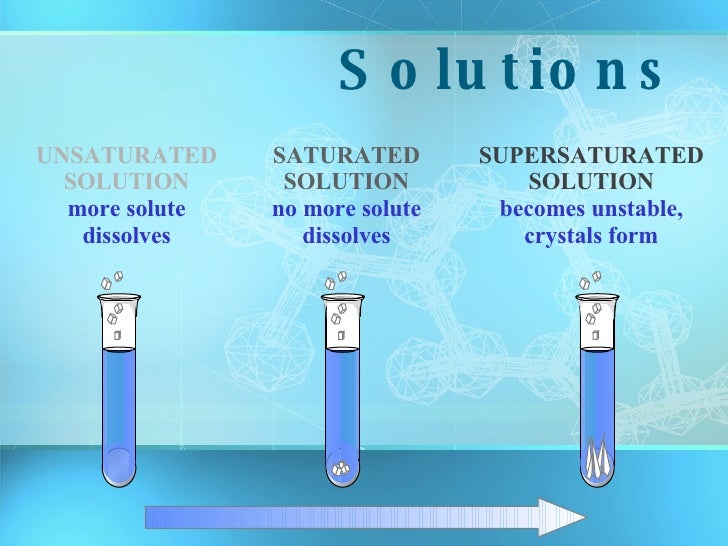
About the saturated and unsaturated. Solutions may be unsaturated, saturated, or supersaturated, depending on the amount. How can you tell if a solution is saturated or unsaturated?
Identify saturated and unsaturated compounds. If more solute is added and it does not dissolve, then the original solution was saturated. How can you tell if a solution is saturated or unsaturated?
151k views 2 years ago. If more solute is added and it does not dissolve, then the original solution was saturated. The figure below illustrates the above process.
A saturated solution is a solution that contains the maximum amount of solute that is capable of being dissolved. How can you tell if a solution is saturated or unsaturated? If more solute is added and it does not dissolve, then the original solution was saturated.
A saturated solution must not be heated as on heating the solution will become unsaturated. If more solute is added and it does not dissolve, then the original solution was saturated. If we next cool the mixture back to 25 °c, 9 g of glucose should precipitate from solution.